Minds On
Particles

Is everything that exists made up of something?
Yes, particles! Particles are tiny bits of matter that make up everything in the universe.
There are many different types of particles, with different particle sizes and properties.
Matter is made up of small particles that are too small to be noticed, even with a powerful microscope. They are so small that you would have to put about 100,000 particles in line to equal the width of a human hair!
Investigate
Investigate

Let’s investigate particles!
Think about what you know and wonder about particles and the particle theory.
Complete the KWL Chart in your notebook or using the following fillable and printable document. If you would like, you can use speech-to-text or audio recording tools to record your thoughts.
|
What I Know What do you think you know about particles or the particle theory? |
What I Wonder What are you wondering about particles or the particle theory? |
What I Learned |
|---|---|---|
Press the ‘Activity’ button to access KWL Chart.
Action
The particle theory

The particle theory of matter is a scientific model used to explain how different types of matter behaves.

Let’s explore the main points of the particle theory:
- all matter is made up of particles
- all particles in a pure substance are identical (no two different pure substances have the same particles)
- all particles have space between them
- all particles are always moving
- all particles are attracted to one another
Types of matter
The three types of matter that are solids, liquids, and gases. The particles that make up each type of matter move differently and are arranged in different ways.
Press the following tabs to explore each type of matter.

In a solid, the particles are packed closely together.
This state of matter has the least amount of energy because the particles are not moving around the space. Instead, they are vibrating in their place.
The density of a solid is high because the forces holding the particles together are strong.
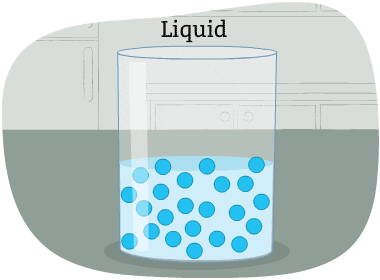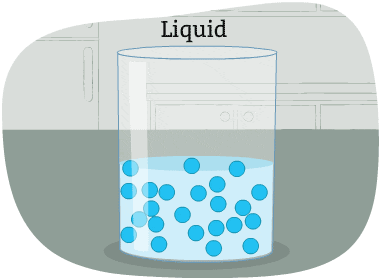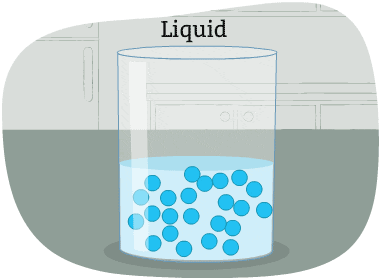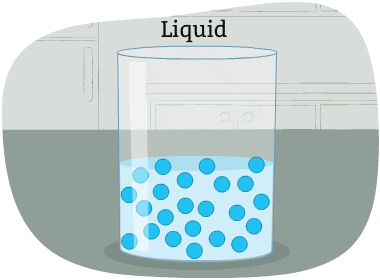
The particles in a liquid are packed closely together, but differently than in a solid.
The forces that hold the particles together are not as strong as in a solid. These particles can move around each other. Their shape is not fixed. So, it changes depending on the container that holds the liquid.
Some liquids are thicker than others, and move more slowly. Liquid particles have more energy than solids.
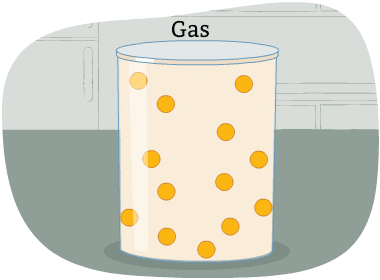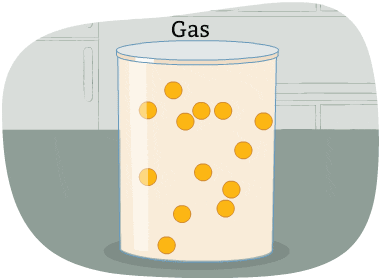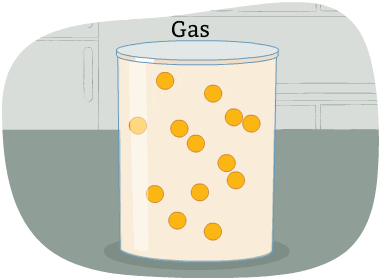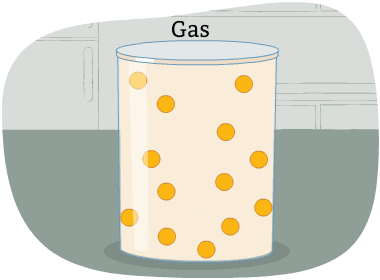
The particles in a gas are far apart from each other.
The energy in these particles is higher than in a liquid and a solid. The particles are always moving in all directions and collide into each other and the sides of the container that holds them. This causes pressure in the container.
A gas is a state of matter with no fixed volume or shape.
In other words, gases take the shape of their container and can be compressed easily.
Try It
Your turn!
If some asked you to explain the particle theory of matter to them, how would you explain it? Would you use words or images?
Record your ideas in a notebook or another method of your choice.
Before beginning the next section, return to the KWL Chart from the Minds On section.
Be sure to add any new information and learning to the chart.
Particles and temperature
So, how does the particle theory explain the behaviour of particles at different temperatures? Let’s find out!
Check out the following Science North video to explore how particles behave when exposed to the very cold temperature of liquid nitrogen.
Explore this video entitled “Particle Theory of Matter” to learn more about how particles behave at different temperatures.
Student Success
Let’s think!
After exploring the video, consider and respond to the following questions:
- What happened to the balloon when it was placed in the liquid nitrogen, which is really cold?
- What happened when the balloon was brought out and back to ‘regular’ temperature?
- What happened to the balloon when it was placed over the beaker that contained liquid nitrogen? Why did this happen?
Record your ideas in a notebook or another method of your choice.
Press ‘Let’s Check!’ to access the correct responses.
1. The balloon began to shrink and fit inside the liquid nitrogen container because the particles of air inside the balloon were getting colder and moving closer together, or losing energy.
2. When the balloon was brought back out to ‘regular’ temperature, it began to expand, or inflate again, because the particles of air gained energy and moved further apart again.
3. When the balloon was placed over the beaker containing liquid nitrogen, the nitrogen particles, which were gaining energy from the heat of their surroundings, began to expand and inflate the balloon because the particles were excited and moving further apart.
Time to experiment!
Before beginning our scientific experiment, explore this video to learn more about the steps of the Scientific Experimentation Process.
Before experimenting, choose one of the two experiment options to explore about how particles react to different temperatures.
Safety
Before you explore the following experiment, let’s perform a safety check.
Hands-on Science
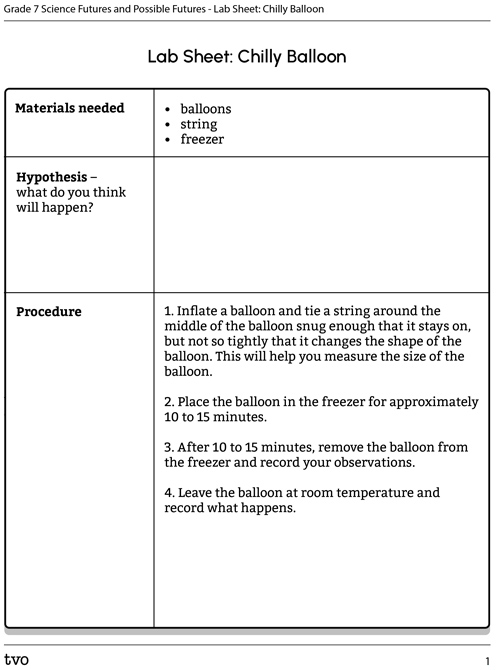
Option A: Chilly balloon
For this experiment, we will be learning about the impact of temperature on particles.
Complete the Lab Sheet: Chilly Balloon in your notebook or using the following fillable and printable document. If you would like, you can use speech-to-text or audio recording tools to record your thoughts.
Press the following tabs to access the materials and procedure for the Chilly Balloon experiment.
If you do not have access to materials, access the “Video demonstration” tab to explore the experiment in action. You can use the video to make your observations and draw your conclusions.
You will need:
- balloons
- string
- freezer
- Inflate a balloon and tie a string around the middle of the balloon snug enough that it stays on, but not so tightly that it changes the shape of the balloon. This will help you measure the size of the balloon.
- Place the balloon in the freezer for approximately 10 to 15 minutes.
- After 10 to 15 minutes, remove the balloon from the freezer and record your observations.
- Leave the balloon at room temperature and record what happens.
Check out this video to explore a demonstration of the Chilly Balloon experiment. Please note that materials and procedure in the video may vary slightly from what is listed in the previous tabs.
When you’re ready, press ‘Let’s Check!’ to access solutions for the follow-up questions.
In this experiment, the balloon had gas particles sealed inside. So, when it was put in the freezer, those gas particles started to lose energy and shrink.
When you leave the balloon out, the balloon warms up and the particles move faster and spread out again, causing the balloon to expand.
Must Have

Science is about reflecting and reimagining. Was your experiment successful?
Is there anything that you would change about your experiment design to improve it or the outcome?
Even if your experiment was not successful, what did you learn or confirm about the topic you were investigating?
Always be sure to do your safety checks before any experiment!
Hands-on Science
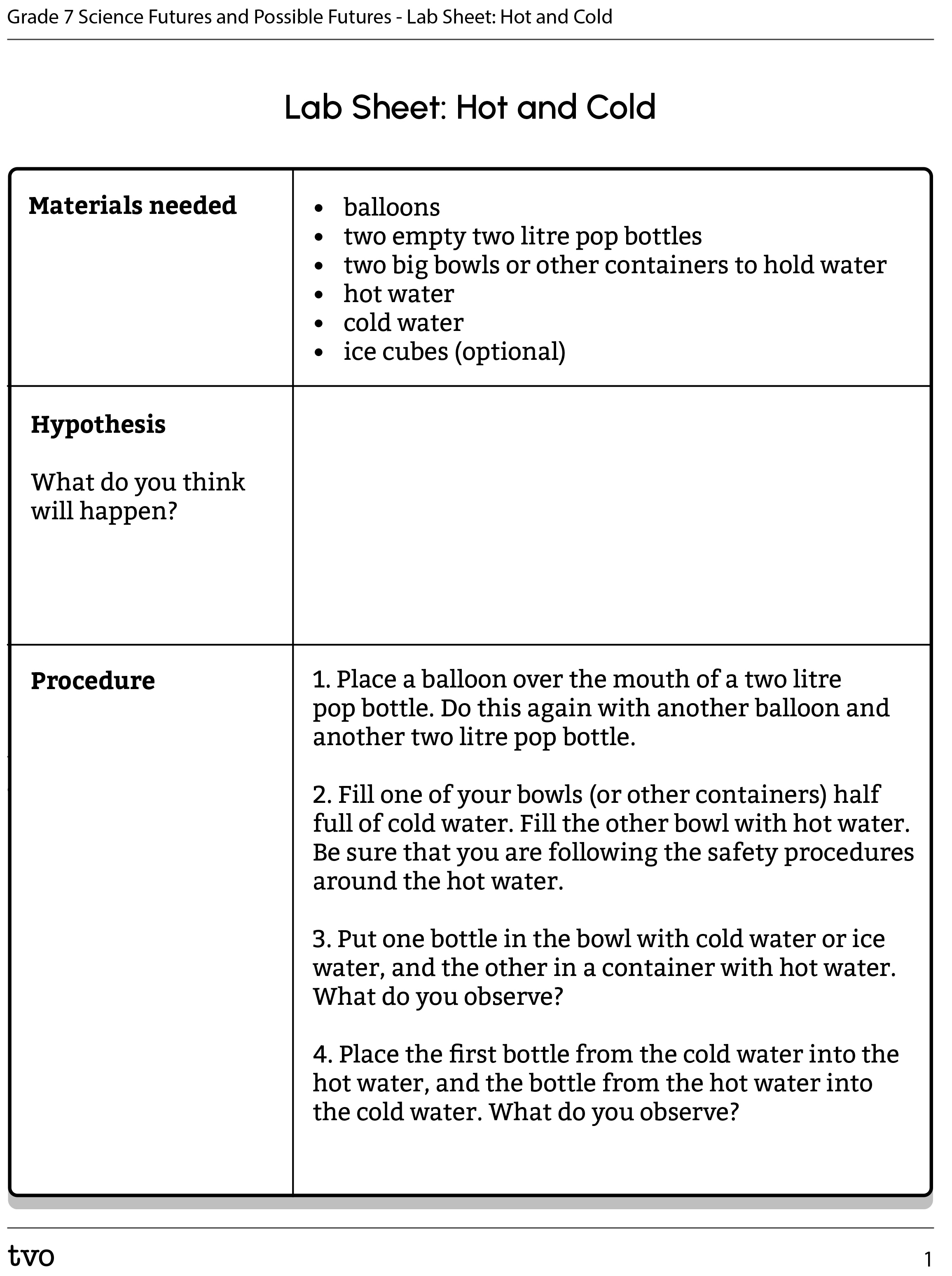
Option B: Hot and cold
For this experiment, we will continue learning about the impact of temperature on particles.
Complete the Lab Sheet: Hot and Cold in your notebook or using the following fillable and printable document. If you would like, you can use speech-to-text or audio recording tools to record your thoughts.
Press the following tabs to access the materials needed and experimental procedure for the Hot and Cold experiment.
If you do not have access to materials, access the “Video demonstration” tab to explore the experiment in action. You can use the video to make your observations and draw your conclusions.
You will need:
- balloons
- two empty two litre pop bottles
- two big bowls or other containers to hold water
- hot water
- cold water
- ice cubes (optional)
- Place a balloon over the mouth of a two litre pop bottle. Do this again with another balloon and another two litre pop bottle.
- Fill one of your bowls (or other containers) half full of cold water. Fill the other bowl with hot water. Be sure that you are following the safety procedures around the hot water.
- Put one bottle in the bowl with cold water or ice water, and the other in a container with hot water. What do you observe?
- Place the first bottle from the cold water into the hot water, and the bottle from the hot water into the cold water. What do you observe?
Check out this video to explore a demonstration of the Hot and Cold experiment. Please note that materials and procedure in the video may vary slightly from what is listed in the previous tabs.
When you’re ready, press ‘Let’s Check!’ to access possible responses for the follow-up questions.
In this experiment, when you place the cold bottle in the hot water, you notice that the balloon expands because the gas particles inside heat up and move.
When you place the bottle in the cold water, the particles slow down causing the balloon to shrink.

Science is about reflecting and reimagining. Was your experiment successful?
Is there anything that you would change about your experiment design to improve it or the outcome?
Even if your experiment was not successful, what did you learn or confirm about the topic you were investigating?
Learning check!
Check your understanding with the following activity.
For each sentence, select the missing word from the drop-down menu.
Future impacts
This learning activity connects new and existing approaches for young scientists to create positive changes in their communities.

Particle physics has changed the way that scientists explore the universe. The use of the particle theory within particle physics has inspired the field of science and improved daily life for people around the world.
“People trained in particle physics develop a unique way of thinking. They know how to approach unknowns, how to solve problems, how to design their own equipment and never give up.”
-Kelen Tuttle, Why Particle Physics Matters

Check out this video entitled “Why Particle Physics Matters” to learn more about particle physics.
Life with particles
Particle physics applies to our daily life in so many valuable ways that we can explore further.
Press the following tabs to discover how particle physics applies to various fields of study.
Particle physics has been responsible for developing new technologies that are used in the medical field to study how particles move, collide, and transform.
This technology is an essential for things like MRI and PET scans.
These tests are used to collect detailed pictures of the human body and discover abnormalities, like diseases or cancer. Many medicines have been developed using the particle theory and particle physics research. It has also been used to develop treatment options for patients.

Many everyday technologies have been developed using particle physics.
For example, semi-conductors within a laptop have been made smaller and faster using particle physics. Examples of this include touch screen computers, tablets, etc.
Another tool that has been developed is called the “scientific linux,” which is an operating system that is used to manage a computer’s programs. It is used by researchers in both laboratories and universities all over the world.

Particle physics is used to learn more about the universe! Particle physicists explore matter that is visible and what makes up the universe on both large and small scales.
They also explore dark matter and energy that makes up majority of our universe, working towards making more discoveries about its origin and what these areas are made of.
They search for undiscovered particles in the universe to try to better understand the how and why of the universe, and to develop new materials and applications. There is still so much yet to be discovered!

Pause and Reflect
Pause and reflect
How do these applications of particle theory impact our lives in the present and in the future?
Record your thoughts in a notebook or another method of your choice.
Consolidation
Review your learning

We have unpacked a lot of valuable information about the particle theory of matter in this learning activity.
Select the correct answer, then press ‘Check Answer’ to see how you did.
Making connections
Recall the KWL Chart from the Minds On section.
Be sure to add any other information you have learned about the particle theory of matter throughout this learning activity.
Reflection
As you read the following descriptions, select the one that best describes your current understanding of the learning in this activity. Press the corresponding button once you have made your choice.
I feel…
Now, expand on your ideas by recording your thoughts using a voice recorder, speech-to-text, or writing tool.
When you review your notes on this learning activity later, reflect on whether you would select a different description based on your further review of the material in this learning activity.