Minds On
Sorting items
Explore the following images:
After exploring the previous images, respond to the following questions:
- Can you think of a time that you needed to separate or sort something? What was it? How did you do it?
- Have you ever needed to separate a mixture?
- Can you think of any examples in real life where you would be required to separate mixtures?
Record your ideas in a notebook or another method of your choice.
Press ‘Hint’ to access a helpful detail.
You may use the images to help guide your responses.
Action
What’s the difference?
Check out this video clip of a scientist mixing two different liquids.
A substance is physical matter or material that has a mass and occupies space. One substance alone cannot be a mixture.
A mixture is the result of two or more substances added together. The substances are not chemically combined and may be separated again. There are homogenous mixtures (solutions) and heterogenous mixtures (mechanical).
Homogenous mixtures are mixtures of two or more substances that cannot be easily separated by common physical means (e.g., settling, filtration, etc.).
Heterogenous mixtures are any combination of substances that do not have uniform composition and properties (mechanical mixtures).
Types of mixtures
Before we learn about separating mixtures, it is important to learn about the different types of mixtures. Try to identify examples of different mixtures.
For each type of mixture, select the corresponding example that matches it.
Many things around us mix naturally, such as salty sea water, soil, compost, or minerals in a rock. Mixtures are not chemically combined and therefore do not turn into new substances – they have just been physically combined. This is why we can use physical methods to separate them.
For example, peanut butter. Justify whether you think peanut butter is a pure substance or a mixture.
Press ‘Hint’ to access an additional detail.

Press the following tabs to access the two videos.
Press ‘Answer’ to access an explanation to check your understanding.

Natural peanut butter is usually made from pure peanuts, and is ground into a creamy butter, which separates out the peanut oil from the thick butter. This can be remixed and is really just pure peanuts. Commercial brands of peanut butter stay as a smooth creamy butter under many conditions, but are actually made from many ingredients into a tasty mixture.
Brainstorm
Brainstorm
Let’s brainstorm a list of everyday pure substances and mixtures you may encounter or use daily.
Record your list in a notebook or another method of your choice.
If possible, compare your list with a partner's.
Separating mixtures
Let’s explore some of the most common ways to physically separate mixtures.
Press the following tabs to access most common ways to physically separate mixtures.
Hand sorting means physically picking up some items from the mixture and separating them. Hand sorting is useful for a mixture that contains a small number of large items. For example, separating types of recyclable materials.
In the following video clip, postal workers are using their hands to sort parcels as they arrive on the conveyer belt.
Reflection
Would hand sorting be a good way to sort rice from beans? Why or why not?

Sieving is used when we have large quantities of materials to sort, and the particles have different sizes. When you sieve a mixture, smaller particles fall through the openings in the sieve, and the larger particles are left behind.
Explore the following video clip of a person using a sieve to separate particles.
Reflection
Can you think of any other examples of sieving?
When the particles of a mixture are too small to be caught by a sieve and/or when they are in different states (solid, liquid, gas), they can be separated using filtration.
Filtration removes tiny pieces of solids from a liquid or gas, as the holes in a filter are usually too small to see.
For example, a coffee filter allows water to pass through, but stops the tiny coffee grinds from passing. It is important to note that the substances that pass through the filter are called the “filtrate” and the particles left behind are called the “residue.”
Access this close-up video clip of coffee grinds in a coffee filter as someone is pouring hot water onto the grids. The water eventually passes through the filter, but the grinds do not.
Reflection
When is filtering a good method for separating mixtures?
Magnetic separation is the process of separating mixtures by using magnets to attract magnetic materials.
For example, magnetic separation may be used to remove specific minerals from substances or when separating other metals.
In the following video clip, mixed glass is being passed on a conveyer belt and any metal pieces are being picked up by the electromagnetic component above the belt.
Reflection
When is magnetic separation a good method for separating mixtures?
Student Success
Think!
If possible, with a partner, organize what we’ve learnt about the most common methods of separating mixtures.
For each process, consider:
- What materials are being used?
- How does it work?
- When should this process be chosen?
- What is an example of this process?
Complete the Separating Mixtures Methods Activity in your notebook or using the following fillable and printable document. If you would like, you can use speech-to-text or audio recording tools to record your thoughts.
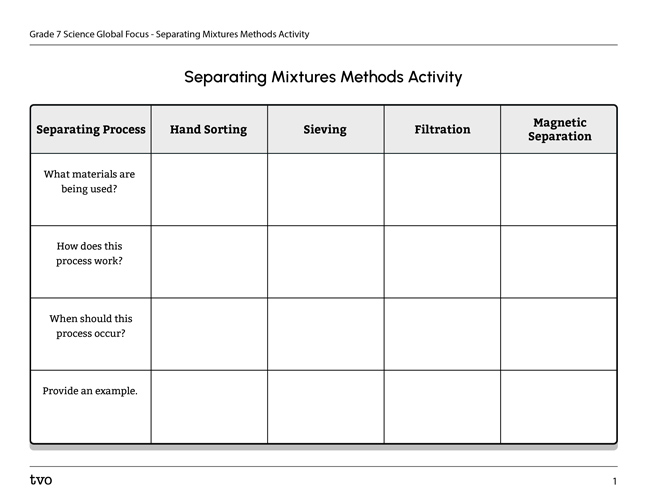
Press the Activity button to access Separating Mixtures Methods Activity.
Activity (Open PDF in a new tab)Note to teachers: See your teacher guide for collaboration tools, ideas and suggestions.
Experimenting with mixtures
You will choose one of the three options to explore separating mixtures further.
Explore this video to learn more about the steps of the Scientific Experimentation Process.
For this activity, we will be focusing on the procedure, materials, observations, and conclusion sections.
Safety
Before you explore the following experiment, let’s perform a safety check.
Hands-on Science
Option A: Using properties to separate mixtures
For this experiment, we will be learning about separating mixtures.
Complete the Lab Sheet: Option A – Using Properties to Separate Mixtures in your notebook or using the following fillable and printable document. If you would like, you can use speech-to-text or audio recording tools to record your thoughts.

Press the Activity button to access Lab Sheet: Option A – Using Properties to Separate Mixtures.
Activity (Open PDF in a new tab)Press the following tabs to access the materials and procedure for the Option A – Using Properties to Separate Mixtures experiment.
You will need:
- mixture of sand, salt, and something small that is magnetic (i.e., paper clip, screw, etc.)
- beaker(s)
- magnet
- plastic bag
- water
- funnel
- filter paper or paper towel
- stirring rod/stir stick/spoon
For this task, develop and design a procedure for separating these substances (sand, salt, magnetic substance) from each other. All three substances must be retrieved from the mixture individually.
Reflect on the following questions:
- Were you able to separate the sand from the salt and the magnetic material?
- What part of the procedure was expected or unexpected?
- How did you separate these three substances? What might you change next time?
Record your ideas in a notebook or another method of your choice.

Science is about reflecting and reimagining. Was your experiment successful?
Is there anything that you would change about your experiment design to improve it or the outcome?
Even if your experiment was not successful, what did you learn or confirm about the topic you were investigating?
Always be sure to do your safety checks before any experiment!
Hands-on Science
Option B: Recycling challenge
Press the following tabs to access the materials needed and experimental procedure for the Option B experiment.
You will need:
- cardboard
- newspaper
- steel cans
- aluminum cans
- glass bottles
- plastic bottles
- various classroom/home supplies (elastics, skewers, popsicle sticks, magnets, tape, pipe cleaner, paper plates, plastic cups, string, paper clips, etc.)
1. Develop a plan that outlines a process for sorting the recycling. Consider what needs to be sorted first, the materials you’ll use, and what you will need to build. Include a drawing of your invention. The primary goal is to invent an efficient recycling system, but the recycling facility is looking for a design that is both efficient and cost effective.
2. Build your recycling system; this step includes any trial runs and modifications.
3. Use your recycling system to sort the components (cardboard, paper, steel, aluminum, glass, plastic, waste).

Reflect on the following question:
- What should someone consider when designing a separating technique for a recycling facility? Why?
Record your ideas in a notebook or another method of your choice.
Complete the Lab Sheet: Option B – Recycling Challenge in your notebook or using the following fillable and printable document. If you would like, you can use speech-to-text or audio recording tools to record your thoughts.
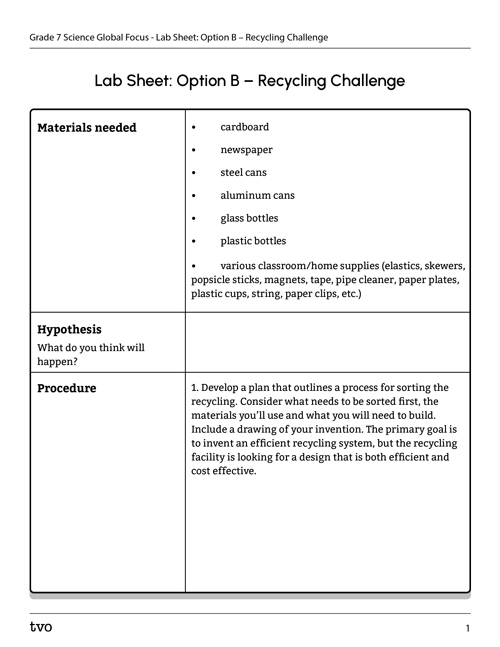
Press the Activity button to access Lab Sheet: Option B – Recycling Challenge.
Activity (Open PDF in a new tab)
Science is about reflecting and reimagining. Was your experiment successful?
Is there anything that you would change about your experiment design to improve it or the outcome?
Even if your experiment was not successful, what did you learn or confirm about the topic you were investigating?
Option 3: Explaining sorting
Press the following tabs to explore each task option to explain sorting.
In detail, describe the process you would design for separating these mixtures (sand, salt, magnetic substance) individually.
What do you perceive would be difficult about this task?
Record your thoughts as a detailed written description, audio recording, or another method of your choice.
Develop a plan that outlines a process for sorting various recyclable materials (i.e., cardboard, newspaper, steel cans, aluminum cans, plastic, glass bottles, etc.).
In detail, describe what materials you would use to build your system and how you would test your system for efficiency.
What do you perceive would be difficult about this task?
Record your thoughts as a detailed written description, audio recording, or another method of your choice.
After exploring the previous task, reflect on the following:
- How do you know if your plan would work?
- What observations would you need to make, or how would you measure success?
Record your ideas in a notebook or another method of your choice.
Global connection

The United Nations (UN) is a group of many countries from around the world that have come together to create a better future for people and the environment. They have created 17 goals called the Sustainable Development Goals.
This learning activity is connected to Goal #11: Sustainable Cities and Communities. This means that our cities and communities should be inclusive, safe, resilient, and sustainable. Communities that are not sustainable can lead to pollution, unrest, insecurity, lack of economic growth, and more.
Let’s take a look at how alternative options for separating mixtures are being explored in an effort to contribute to this development goal of sustainable cities and communities.
Sustainably separate mixtures

Various industries separate mixtures to produce pure products. In this component of the learning activity, you will learn about evaporation and distillation as two different ways to separate mixtures, understand how these methods are used in the real world, and explore sustainable options for separating mixtures.
Press the following tabs to access various ways to separate mixtures.
Evaporation occurs when a liquid changes into a gas and the solid is left behind. This process is often used to remove liquid from a solution made of a liquid and solid.
For example, through the process of making maple syrup, evaporation takes place.
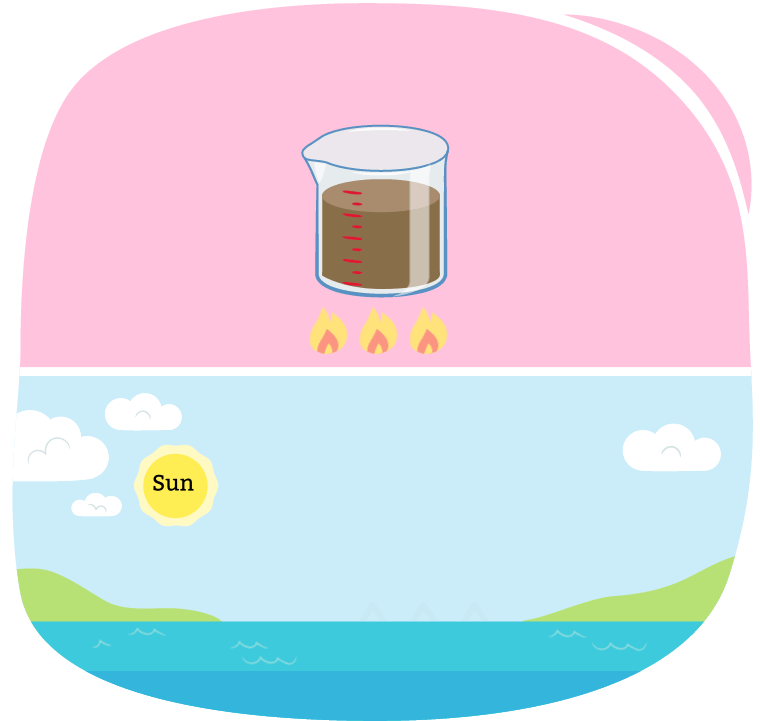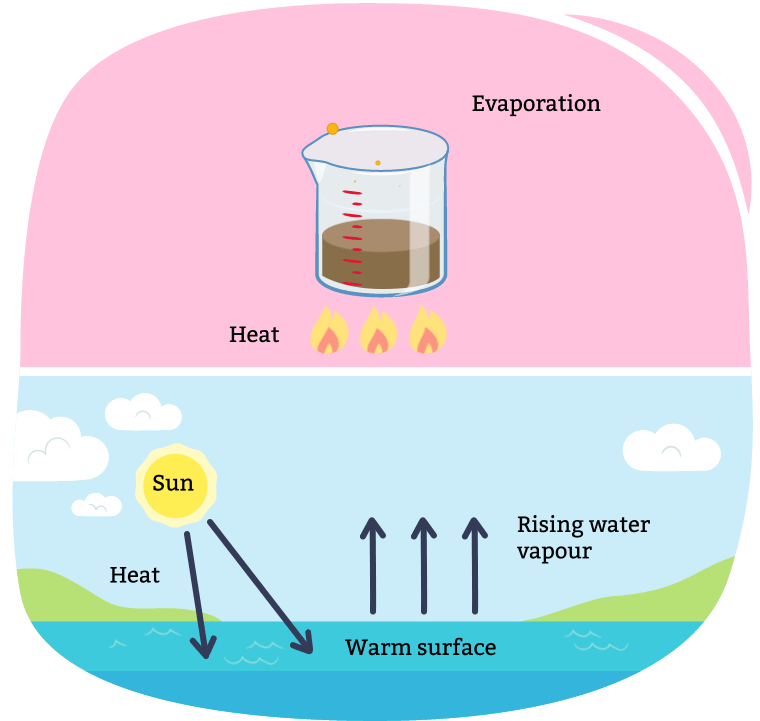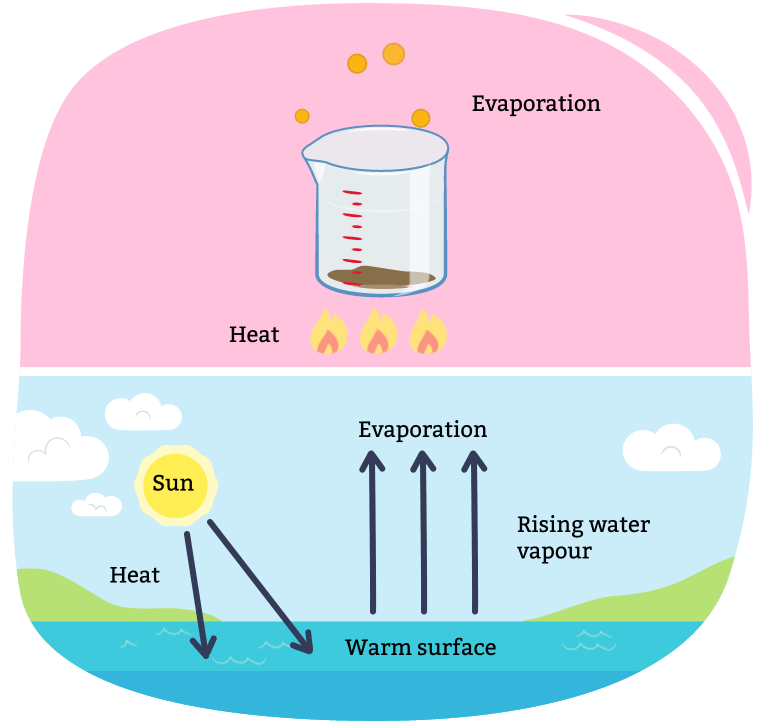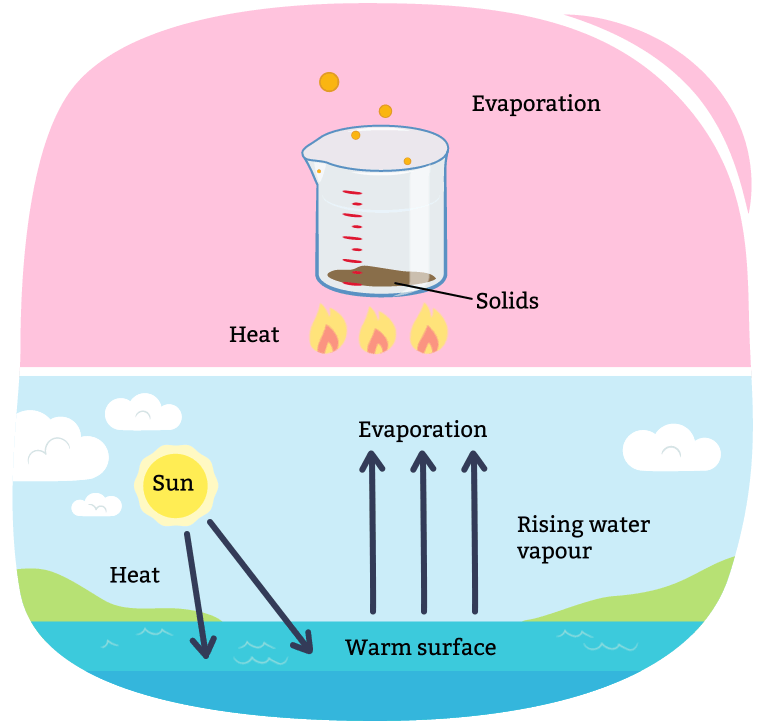 Description
Description
A diagram illustrating evaporation. Evaporation can occur over time, or be sped up through boiling the mixture with a heat source. When all the liquid is evaporated, you are left only with the solids. Evaporation also occurs in our natural environment every day with the Sun. As the sun heats up water (i.e. a lake, puddle, and snow), it evaporates into the air, turning into a gas.
Distillation is the process of separating liquids in a solution by heating the solution, trapping and cooling the gas, and collecting the resulting pure liquid.
If you had a liquid solution with more than two different liquids, you could use the distillation process more than once to separate all of the liquids.
For instance, distillation takes place through the process of making distilled (pure) water.
 Description
Description
A distillation setup to change saltwater to distilled water. A container with saltwater is placed over a Bunsen burner, then steam travels upward and into a Lieberg condenser where its surrounded by cooling water. From there, the condensed water is deposited into a receiving flask as distilled water.
Sustainable methods

One of the main considerations of sustainable separation techniques is to consider how much energy is being used during the separation process. Energy and cost-efficient technologies are being developed to separate chemicals, and chemical engineers are working to find alternatives to distillation.
Discovery and process scientists, with chemical engineers, are working toward improving separation techniques based on the separation of molecules.
These sustainable alternatives to distillation for fluid separations will be a game-changer for global communities for the following reasons:
- They will reduce the amount of energy consumed by the distillation process.
- It will improve cost and environmental performance of chemical separation.
- It will allow new technologies to be implemented, which will generate high-skilled jobs.
- Similar technologies can be applied to related sectors, such as bio-based and renewable chemical production.
Pause and Reflect
Pause and reflect
How do you think these new technologies and techniques help the UN achieve Goal #11?
Record your ideas in a notebook or another method of your choice.
Consolidation
Create your own procedure

Imagine you are a member of a team of scientists.
You’ve been given a mixture that contains sand, iron, salt, ethanol, and water.
Your task is to design a sustainable procedure to separate the mixtures into individual components. How would you do that?
Use the following checklist to help you design a procedure to separate the mixture.
Procedure Design Checklist
You can design the procedure by…
You may summarize your ideas and outline each step for the separating procedure in a notebook or another method of your choice.
If possible, try to create your own demonstration of this separation technique to share with others using a video or audio recording.
Reflection
As you read the following descriptions, select the one that best describes your current understanding of the learning in this activity. Press the corresponding button once you have made your choice.
I feel…
Now, expand on your ideas by recording your thoughts using a voice recorder, speech-to-text, or writing tool.
When you review your notes on this learning activity later, reflect on whether you would select a different description based on your further review of the material in this learning activity.


