Minds On
Examining
Examine the two glasses of water in the following image.

Pause and Reflect
Pause and reflect
What do you observe, infer, and wonder about as you examine the photo?
Record a few ideas for each of the following sentence fragments in a notebook or using another method of your choice. If you would like, you can use speech-to-text or audio recording tools to record your thoughts.
- I observe… (record points you can observe with your senses)
- I think… (record what you think from what you’ve noticed and what you might already know)
- I wonder… (record questions you may have)
Action
Global connection

The United Nations (UN) is a group of many countries from around the world that have come together to create a better future for people and the environment. They have created 17 goals called the Sustainable Development Goals.
This learning activity is connected to Goal #6: Clean Water and Sanitation. This means everyone should have Safe drinking water and sanitation. Having clean water affects not only health but things like reducing poverty, having food security, and having a sustainable ecosystem.
What affects water quality?

Typically, water quality is determined by comparing the physical and chemical characteristics of a water sample with quality guidelines or standards. High-quality water is water that is clean. It is not contaminated by dirt, chemicals, or pollution, and it is safe for drinking. Declining water quality has become a global issue of concern as human populations grow, industrial and agricultural activities expand, and climate change threatens to cause major alterations to the water cycle.
Eutrophication
Globally, the most prevalent water quality issue is “eutrophication.”
Press Eutrophication to access a definition of eutrophication.

Eutrophication results from products that are high in nutrients like phosphate entering the water from farmland run-off, sewage, industrial plants, and the burning of fossil fuels. When there are too many nutrients in a lake or other body of water, plants and algae grow very densely and use up the oxygen in the water. Eutrophic water smells bad, is no longer transparent, and it is hard for life to survive there.
Personal Care Products

Another emerging concern is the impact of personal care products (for example, shampoo and medications) on water ecosystems. Water quality concerns limit the amount of water that can be used for drinking and bathing.
Pollution

There are two types of pollution that are impacting water systems globally. Press the following tabs to find out more about these two types of pollution.
As runoff water from rainfall or melting snow moves through the ground, it picks up and carries natural and human-made substances such as chemical sediments and debris. The runoff water then carries these substances into lakes, rivers, and wetlands.
Climate Change

There are three images, the image on the left is of a dark sky, a bare tree and dry cracked ground. The middle image is of a flood with a chair, bucket, and trailer floating in the water. The image on the right is of a tornado.
Another major factor that is impacting water quality around the world is climate change. Climate change is causing more severe droughts and floods in different areas in the world. Climate scientists predict that this shift will lead to more floods, since more water will fall than vegetation and soil can absorb. The remaining water, or runoff, drains into nearby waterways, picking up contaminants like fertilizer on the way. Excess runoff eventually travels to larger bodies of water (like lakes, estuaries, and the ocean), polluting the water supply and limiting clean water access for humans and ecosystems.
Pause and Reflect
Pause and reflect
- What factors are impacting global access to safe water?
Record your ideas using a method of your choice.
Testing Water Quality
Connections
Career profile: Water quality technician

A water quality technician is responsible for monitoring and operating systems to ensure water treatment and filtration plants are operating correctly. They inspect, sample, monitor, and test various water sources, as well as addressing complaints of water quality issues. Water quality technicians spend time in the field, the lab, and in an office depending on the task they are working on. The role of a water quality technician is essential in ensuing water for humans and our environment is safe.
Let’s Experiment!

Today, you will be thinking like a water quality technician! There are two different options for you to choose from.
- Option 1 has you explore the pH scale using an interactive simulation.
- Option 2 has you creating and exploring jars of mixtures and determining how you would measure water quality.
If time allows, you may wish to do both!

A pH scale. The scale starts at 0 and goes up to 14. 0 being the most acidic, 7 being neutral and 14 being the most basic. The examples of the scale include battery acid/hydrochloric acid pH of 0, lemon juice/vinegar pH of 2, grapefruit juice/soda/tomato juice pH of 2.5 to 3.5, black coffee pH of 5, milk pH of 6.3 to 6.6, blood pH of 7.4, sea water pH of 8, baking soda pH of 9.5, ammonia solution pH of 10.5 to 11.5, bleaches/oven cleaner/lye pH of 13.5, and liquid drain cleaner pH of 14.
Before you begin, you’ll need to understand what pH means. A liquid’s pH is a measure of how acidic or basic it is. The range goes from 0 to 14, with 7 being neutral. A pH lower than 7 indicates an acidic liquid (an acid), whereas a pH of greater than 7 indicates an alkaline liquid (a base). The pH level is an important quality that reflects the chemical conditions of a solution and therefore is helpful in determining if water is safe to drink. The pH of water also affects living organisms in the water and can be an indicator of increasing pollution or other environmental factors.
Option 1: pH Scale Basics simulation
Access the following interactive activity entitled pH Scale Basics to explore the pH level of various substances.
Note that this simulation is not optimized for non-visual learners. If you are unable to interact with the following simulation, you can complete Option 2: Experimentation activity instead.
Press ‘Let’s Check!’ to access key tips for using the virtual simulator.
This interactive simulation explores the pH of various everyday liquids and how pH is affected when you dilute a liquid with water. Here’s how to get started:
- Choose an everyday liquid from the drop-down menu at the top of the screen. The eye dropper will fill up with that liquid.
- Next, press the red dot on the eye dropper to add some of the everyday liquid to the beaker. You can add as much or as little liquid as you wish.
- Drag the green target into the liquid and record the pH measurement in your notebook, using the fillable and printable document pH Level Tracking Organizer, or using another method of your choice.
What do you think will happen if you add water to the everyday liquid? Will the pH increase or decrease?
- Press the blue knob on the water tap to dilute the liquid and create a solution. Explore how the pH changes as you add more water to the solution, or as you add more of the everyday liquid to the solution. Record your observations.
You can press the blue knob on the drainpipe to empty the beaker and start again with a new liquid. Explore all the everyday liquids and how their pH changes when you dilute them with water. Don’t forget to record your observations!
Record your observations in the pH Level Tracking Organizer provided, or using another method of your choice. In this interactive simulation, you can explore various substances and test their pH level by dragging the green target into the solution.
Access the following fillable and printable document pH Level Tracking Organizer to complete the activity.
| Substance | pH Level |
|---|---|
| (For example: distilled water is pH 7) | 7 |
Press the ‘Activity’ button to access pH Level Tracking Organizer.
What are your conclusions?

Draw conclusions about this experiment by answering the following questions in your notebook or using another method of your choice.
- Which substances had very high pH levels? Which substances had very low pH levels? What does this tell you?
- What happened to the pH levels when you added water to substances?
- Why is it important to consider pH levels when testing water?
Check your work against the supplied answers. When you are ready, press Answers to learn the suggested observations and conclusions you should have made.
- High pH level liquids include drain cleaner and hand soap. Low pH level liquids include battery acid, chicken soup, coffee, OJ, Soda Pop, and vomit. Middle pH level liquids (around 7) include blood, milk, saliva/spit, and water.
- When water was added to substances, the pH level of substances that were initially low began to increase. The substances with high pH levels to start with began to lower.
- It is important to consider pH levels when testing drinking water because it identifies if there are any harmful chemicals or pollutants in the water that make it unsafe to drink.
Option 2: Experimentation
Safety
Before you explore the following experiment, let’s perform a safety check.
Hands-on Science
Determining water quality, part 1
For this experiment, you will be creating, observing and comparing different water samples to begin to establish criteria for assessing water quality.
Press the following tabs to access the materials and procedure for the Determining water quality, part 1 experiment.
- 4 jars or glasses
- Water from your local river, stream, or lake if possible. You can also use tap water.
- Cocoa powder or coffee grounds
- Food colouring
- Kosher salt or regular salt
- A tablespoon
To begin this experiment, you will need to prepare 4 jars or glasses of water as follows.
- Fill all four jars with water from your local river, stream, or lake if possible. You can also use tap water.
- Take the first jar and add enough cocoa powder or coffee grounds so that the water appears “dirty.”
- Take the second jar and add several drops of food colouring.
- Take the third jar and add a few tablespoons of kosher salt or regular salt.
- You will not add anything to the fourth jar.
- Carefully observe all four jars and record your observations.
Complete Lab Sheet: Determining water quality, part 1 in your notebook or using the following fillable and printable document. If you would like, you can use speech-to-text or audio recording tools to record your thoughts.
|
Ask a Question |
How can we determine water quality? |
|
Materials needed |
|
|
Hypothesis A hypothesis is a possible answer to a question or explanation that accounts for all the observed facts and is testable. |
Ways we can determine water quality are… (Blank) (Blank) (Blank) |
|
Safety |
Follow the safety considerations mentioned earlier in this learning activity. |
|
Procedure |
Prepare 4 jars or glasses of water as follows.
|
|
Observations Record your observations of the jars. Include a diagram that is labelled and what you physically observe when exploring the jars of water. *Remember observations can be recorded with pictures, numbers and/or words! |
 (Blank) (Blank)
(Blank) (Blank) (Blank)
(Blank)
|
|
Conclusions |
1. Which jars would you be willing to use for: fishing, boating, swimming, and drinking? (Blank) (Blank) (Blank)2. How would you test the water quality of these jars? (Blank) (Blank) (Blank)3. Why is it important to care about water quality? (Blank) (Blank) (Blank) |
Press the ‘Activity’ button to access Lab Sheet: Determining water quality, part 1.
Hands-on Science
Determining water quality, part 2
Now you will explore an experiment for testing the pH of various sources of water.
Complete the Lab Sheet: Determining Water Quality, Part 2 in your notebook or using the following fillable and printable document. If you would like, you can use speech-to-text or audio recording tools to record your thoughts.
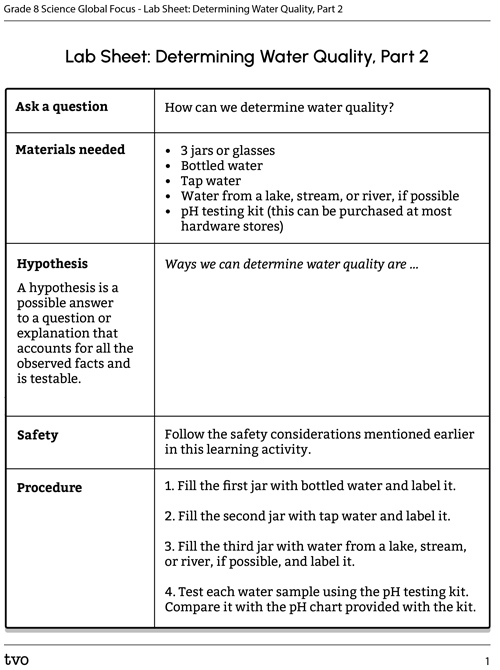
Press the Activity button to access the Lab Sheet: Determining Water Quality, Part 2.
Activity (Open PDF in a new tab)Press the following tabs to access the materials and procedure for the Determining water quality, part 2 experiment.
If you do not have access to materials, access the “Video demonstration” tab to explore the experiment in action. You can use the video to make your observations and draw your conclusions.
- 3 jars or glasses
- Bottled water
- Tap water
- Water from a lake, stream, or river, if possible
- pH testing kit (this can be purchased at most hardware stores)
- Fill the first jar with bottled water and label it.
- Fill the second jar with tap water and label it.
- Fill the third jar with water from a lake, stream, or river, if possible, and label it.
- Test each water sample using the pH testing kit. Compare it with the pH chart provided with the kit.
- Record your observations. Be sure to include levels of iron, nitrates, pH, and chlorine if possible.
Check out this video to explore a demonstration of the Determining water quality, part 2 experiment. Please note that materials and procedure in the video may vary slightly from what is listed in the previous tabs.
Why care?
Water testing can make you a citizen scientist! Take a moment to pause and reflect.
Pause and Reflect
Pause and reflect
After exploring the following image, do you feel the water tested in the video is safe for humans and animals? Why or why not? Record your ideas in a method of your choice.

There is a pH scale. The scale starts at 0 and goes up to 14. 0 being the most acidic, 7 being neutral and 14 being the most basic. Within the pH range of 0 to 4 conditions are too acidic for fish, and between the pH range of 11 to 14 conditions are also unsafe because they are too basic. Healthy conditions for fish are between the pH of 6.5 and 9. Healthy conditions for crabs, snails and mussels is between a pH of 7.5 and 10. Plants favour conditions with a pH between 9 to 12.5. Healthy pH conditions for humans is between 4 to 11, and blood has a pH of about 7.5. For natural conditions, acid rain is classified with a pH of 0.5 to 5. Precipitation has a pH of 5, steam has a pH of 6 to 7.5. Sea water has a pH of 8.
So, why do we care? Well, there a lot of consequences if pH levels are not maintained. When pH levels are too low, fish become prone to infections or other physical damage and an inability to have growth develops in aquatic systems. Many fish will leave the area and or could possibly die if the pH levels fall below 4. If pH levels are too high (over 9), ammonia is released into the water, which will trigger the growth of moss and algae. If algae blooms grow too large, they can kill the ecosystem.
What causes changes to pH levels?
- Acid Rain is caused by specific chemicals being released into the air. Generally, chemicals that results from coal or fossil fuel burning increase levels of sulfur dioxide and oxides of nitrogen which results in acid rain. Acid rain causes a significant change in pH levels of lakes and oceans.
- Natural Process can cause a change in pH levels. Such as: weathering of rocks, leaching from soil, and evapotranspiration due to wind can cause a change in pH levels.
- Fertilizer run-off from farms can change the pH levels in major waterways.
- An increase in temperature (caused by global warming) changes the pH levels in water.
Consolidation
Check my understanding!
Select the correct answer, then press “Check Answer” to see how you did.
Water Testing Guide
Student Success
Think!

If possible, work with a partner or a group to create your own water testing guide for someone who has never tested the quality of water before. What important information would you include? Can you connect this to the career you learned about in this module?
Create your guide using a method of your choice. Some ideas include creating a poster, recording a video, or delivering an oral presentation to share your knowledge.
Note to teachers: See your teacher guide for collaboration tools, ideas and suggestions.
Reflection
As you read the following descriptions, select the one that best describes your current understanding of the learning in this activity. Press the corresponding button once you have made your choice.
I feel…
Now, expand on your ideas by recording your thoughts using a voice recorder, speech-to-text, or writing tool.
When you review your notes on this learning activity later, reflect on whether you would select a different description based on your further review of the material in this learning activity.