Minds On
Water
Check out the following video of flowing water.
Brainstorm
Brainstorm
Let’s brainstorm what we know about water.
Consider the following:
- What do you use water for in your everyday life?
- What else is dependent on water?
- What would happen if there was no more water?
Complete the Water Brainstorm Activity in your notebook or using the following fillable and printable document. If you would like, you can use speech-to-text or audio recording tools to record your thoughts.
Action
Water as a solvent

In this learning activity, you will be exploring water as a universal solvent. A solvent is a liquid that can dissolve another substance.
What is water?
Water is a clear liquid that has no odour and is almost colourless. It is essential for all living organisms.
Water is important because it has many important functions include being a good solvent for dissolving many solids.
Did You Know?
Did you know?
So what does “water is the universal solvent” mean?
Universal means that something is applicable everywhere, or generally appliable in most cases.
A solvent is any material that dissolves another substance.
Therefore, a universal solvent is something that dissolves another substance, also known as a solute, in most cases. Water is capable of dissolving more substances than any other liquid!
Dissolving
Have you ever seen a powdered drink mix with water (or watched someone else do it) , like lemonade, green tea, instant coffee, matcha, or simply sugar and water? What happens to the powder?
Check out the following video of mixing instant coffee into water.
When water and powder are mixed together, the powder is completely dissolved. What is created is called a solution.
The particles of the solid still exist, they simply separate from each other. They become microscopic in the water and can no longer be detected.
Factors
We know that water can dissolve almost any substance, but there are factors that affect a substance’s ability to dissolve.
Press the following tabs to learn about the different types of factors.
The particle size is a factor. The smaller the particle, the easier it is to dissolve.
Temperature of the water is another factor for dissolving a substance. Higher temperatures make it easier to break the bonds between the particles in a substance, because particles move faster in higher temperatures.
If the solution is stirred, it brings the water into contact with the substance, which causes collisions of the particles.
The solubility of the substance is an important factor. Some substances have higher solubility than other substances, which is determined by how much of the substance can be dissolved in the water.
Let’s keep a few key terms in mind as we work through the upcoming experiment.
Before you begin, press ‘Key Terms’ to review definitions of key terms used in the experiment.
- A solute is the substance that is dissolved in a solvent.
- A solvent is the substance that dissolves the solute.
- Soluble means that a substance can be dissolved. The factors that determine if a substance is soluble in water are particle size and temperature (higher temperatures means a higher solubility).
- Insoluble means that a substance is incapable of being dissolved.
Let’s experiment!
Before beginning the experiment, let’s explore this video to learn more about the steps of the Scientific Experimentation Process.
Safety
Before you explore the following experiment, let’s perform a safety check.
Hands-on Science
Dissolving with water
For this experiment, we will be learning about how different substances dissolve in water.
Press the following tabs to access the materials and procedure for the Dissolving Substances in Water experiment.
If you do not have access to materials, access the “Video demonstration” tab to explore the experiment in action.
You can use the video to make your observations and draw your conclusions.
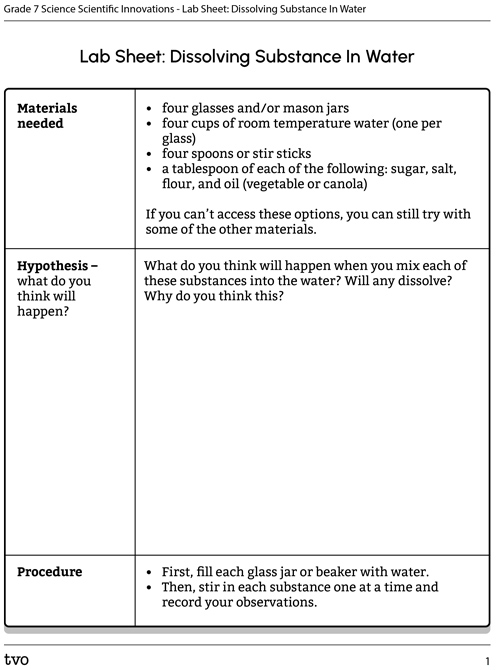
You will need:
- four glasses and/or mason jars
- four cups of room temperature water (one per glass)
- four spoons or stir sticks
- a tablespoon of each of the following: sugar, salt, flour, and oil (vegetable or canola)
If you can’t access these options, you can still try with some of the other materials.
- First, fill each glass jar or beaker with water.
- Then, stir in each substance one at a time and record your observations.
Check out this video to explore a demonstration of the Dissolving Substance in Water experiment. Please note that materials and procedure in the video may vary slightly from what is listed in the previous tabs.
Complete the Lab Sheet: Dissolving Substances in Water in your notebook or using the following fillable and printable document. If you would like, you can use speech-to-text or audio recording tools to record your thoughts.
Press the Activity button to access the Lab Sheet: Dissolving Substance In Water.
Activity (Open PDF in a new tab)Press ‘Conclusion’ to access the final results and conclusions based off of the explored experiment.
In conclusion, the particle charge that develops across the water molecule helps to make it an excellent solvent.
Water dissolves many substances by pulling charged particles into a solution. It does this by surrounding those particles.
Water has “polar molecules.” This means that water has a positive molecule at one end, and a negative molecule at the other end. Polar molecules are ready to react and mix with other substances, therefore making it the universal solvent.

Science is about reflecting and reimagining. Was your experiment successful?
Is there anything that you would change about your experiment design to improve it or the outcome?
Even if your experiment was not successful, what did you learn or confirm about the topic you were investigating?
Consolidation
Review your learning

This learning activity thoroughly explored new vocabulary!
For each ‘term’, select the corresponding definition.
Pause and Reflect
Pause and reflect
Let’s reflect on how you can explain and describe the following:
Why is water the universal solvent?
Record your ideas in a notebook or another method of your choice.
Consider the following:
- an explanation of “why water is the universal solvent”
- key terms and definitions to support your ideas
- an illustration, image, or diagram
- the factors that affect a substance’s ability to dissolve in water
- examples or conclusions from the previously explored experiment in the Action section
You may use the following checklist to guide how you can communicate your description.
I can communicate learning by…
If possible, share your description with a partner.
Reflection
As you read the following descriptions, select the one that best describes your current understanding of the learning in this activity. Press the corresponding button once you have made your choice.
I feel…
Now, expand on your ideas by recording your thoughts using a voice recorder, speech-to-text, or writing tool.
When you review your notes on this learning activity later, reflect on whether you would select a different description based on your further review of the material.