Minds On
All about matter

A person standing under some clouds with dots in them. Under the person it is labeled gas. A person sitting with an umbrella while it rains, Under the person it is labeled liquid. A person standing next to a snowman with a snowflake overhead. Under the person it is labeled solid.
Everything in our lives is made up of matter. Matter is any substance that has mass and takes up space. Matter exists in various states. The most common states are solids, liquids, and gases. Let’s review what each of these are before we begin.
A solid is a state of matter with a fixed shape and volume.
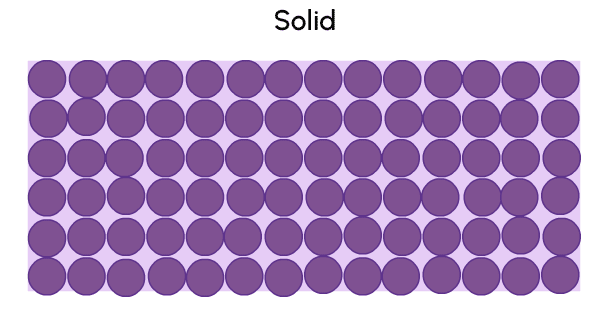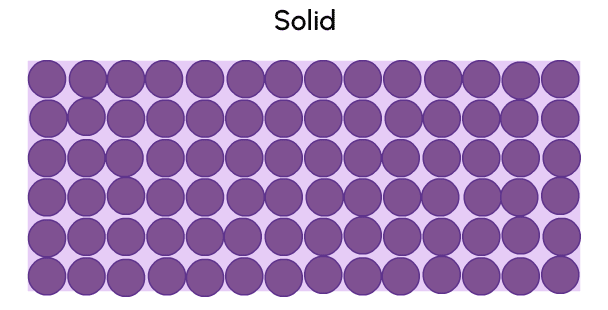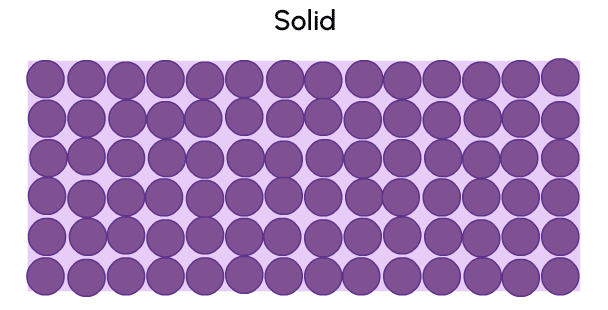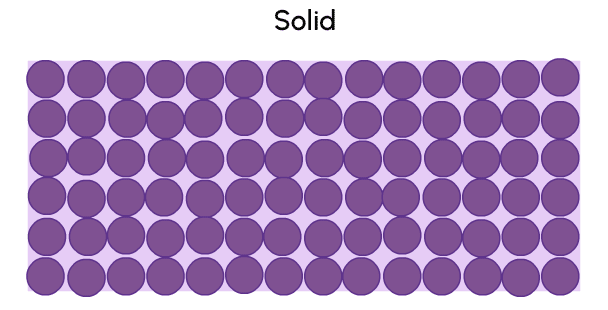
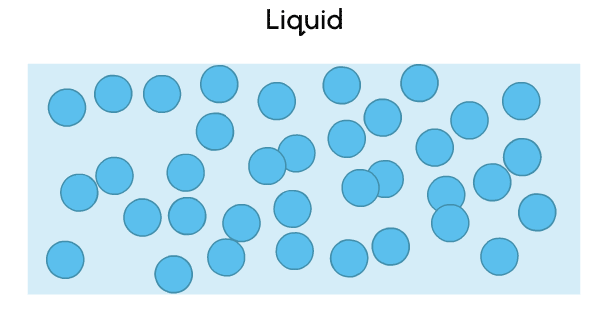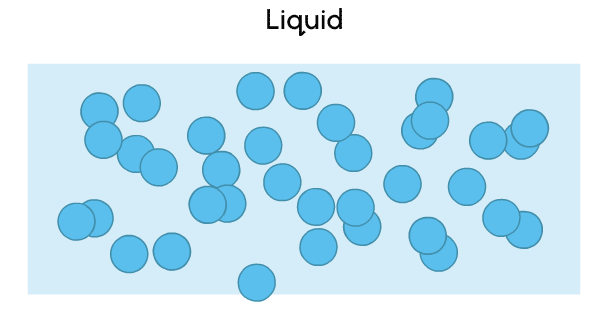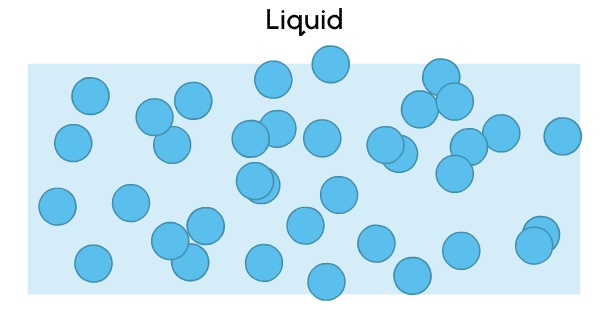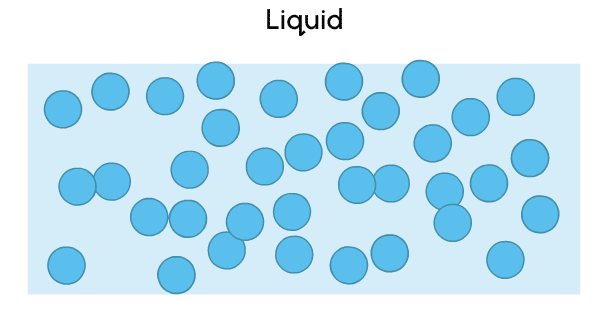
A liquid is a state of matter with no fixed shape, but a fixed volume.
A gas is a state of matter with no fixed volume or shape that can be more easily compressed than solids or liquids.
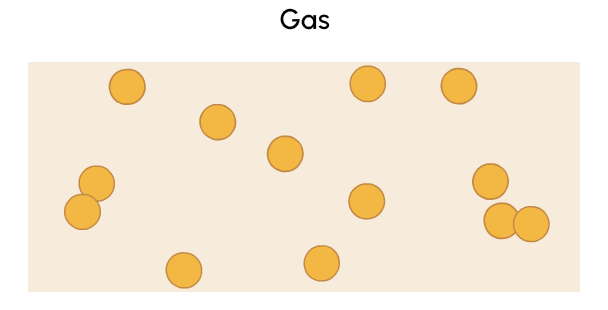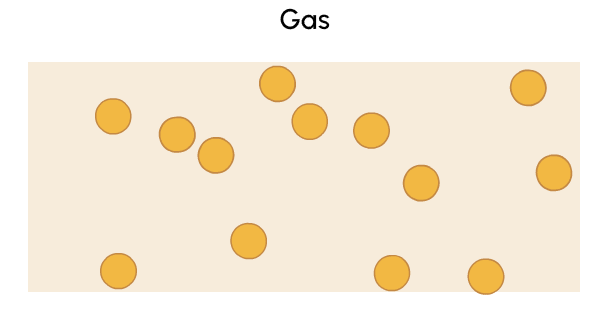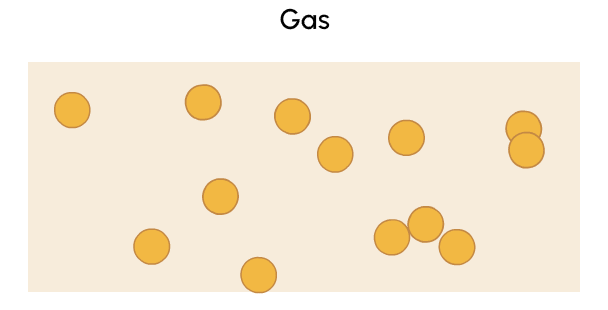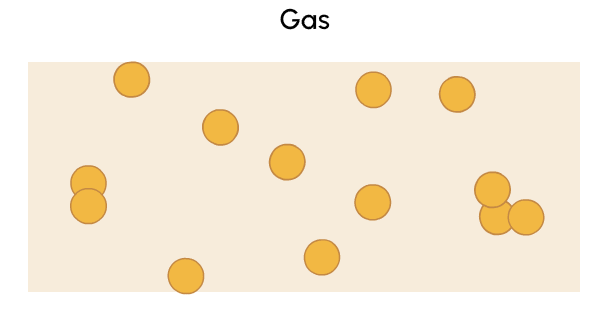
Which category do you think each of these items belongs to: solid, liquid, or gas?
Sometimes matter can change states. For example, something could change from a solid to a liquid, or from a liquid to a gas. What do you think causes matter to change states? Brainstorm your ideas in writing, orally, in print, or in another method of your choice.
Press ‘Hint’ to access a hint about states of matter.
Think about an ice cube. What might cause it to change states? What are those states?
Action
Everyday states of matter

There are many examples of physical changes in states of matter that we explore every day.
Let’s imagine it is a very hot day and Learner A is enjoying a popsicle. What might happen if Learner A does not eat it fast enough? Why?
Press ‘Answer’ to learn what will happen.
The popsicle will melt!
The heat from the sun will cause Learner A’s popsicle, which was a solid, to melt into a liquid!
The changes matter!
Let’s consider how water changes from state to state. These changes are all physical changes.
Press the following tabs to access the changes in states of matter.
Melting is the change of state from a solid to a liquid.
So, how else might a solid change into a liquid?
In the winter season, different places can get lots of ice and snow. What do you think happens as the temperature begins to rise in the spring?
As the temperature rises, the heat causes the ice and snow to melt creating water (liquid)!
Freezing is the change of state from a liquid into a solid.
Let’s say it was a hot summer’s day. If someone wanted a cold drink, what could they do?
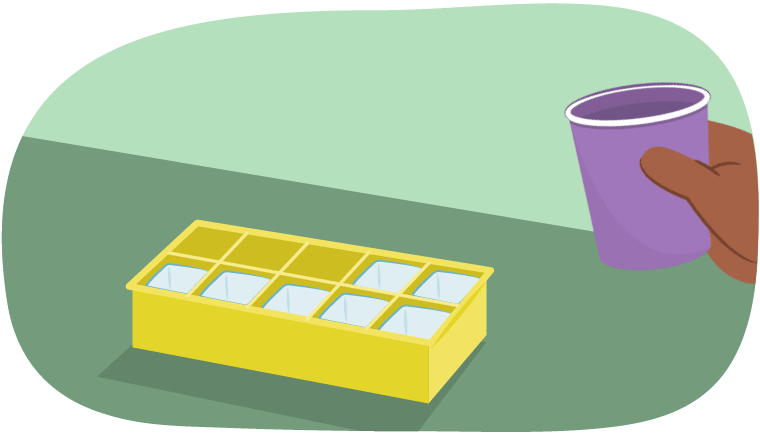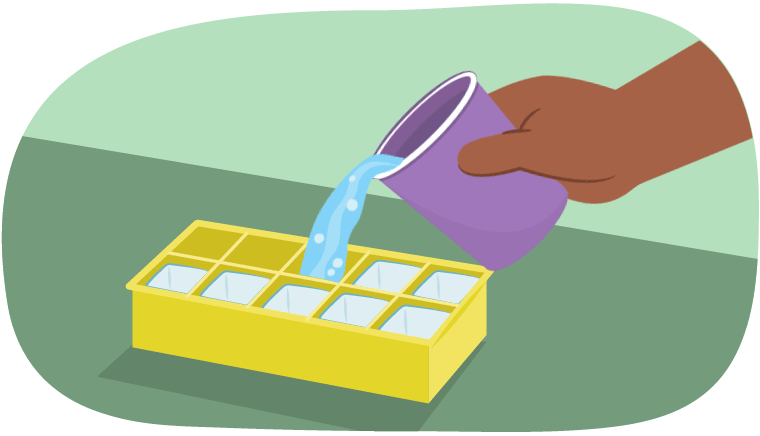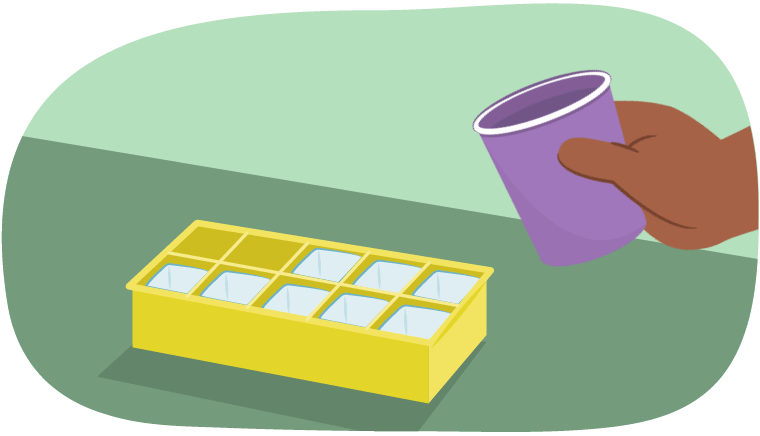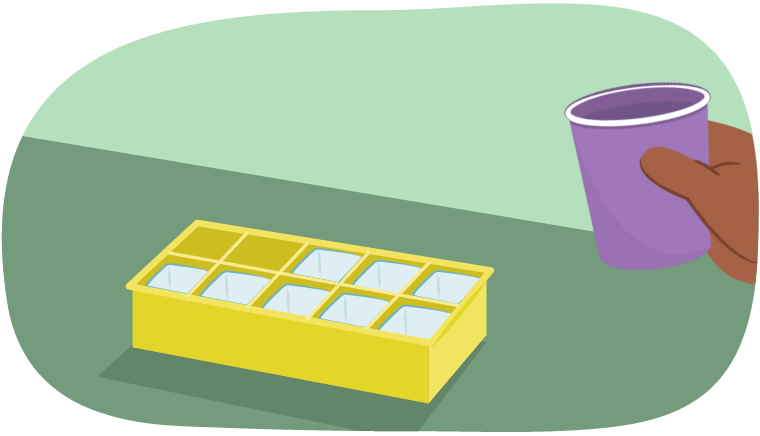
They might create ice! To do this, they would need to pour water into an ice cube tray and putting the tray into the freezer.
When something freezes, it loses some of its heat and its temperature goes down. Water freezes at 0 degrees Celsius.
Now the question is, can a liquid change into a gas?
Yes! And we have explored this change of state of matter around us many times but probably never even noticed it.
Imagine Learner B is making a pot of rice. How do you they know when to add the rice to the pot?
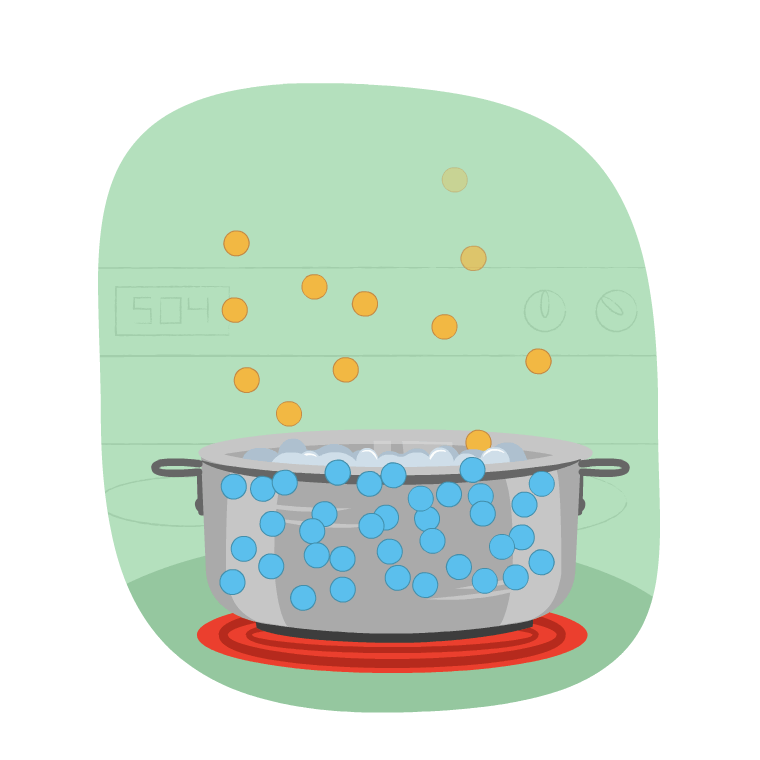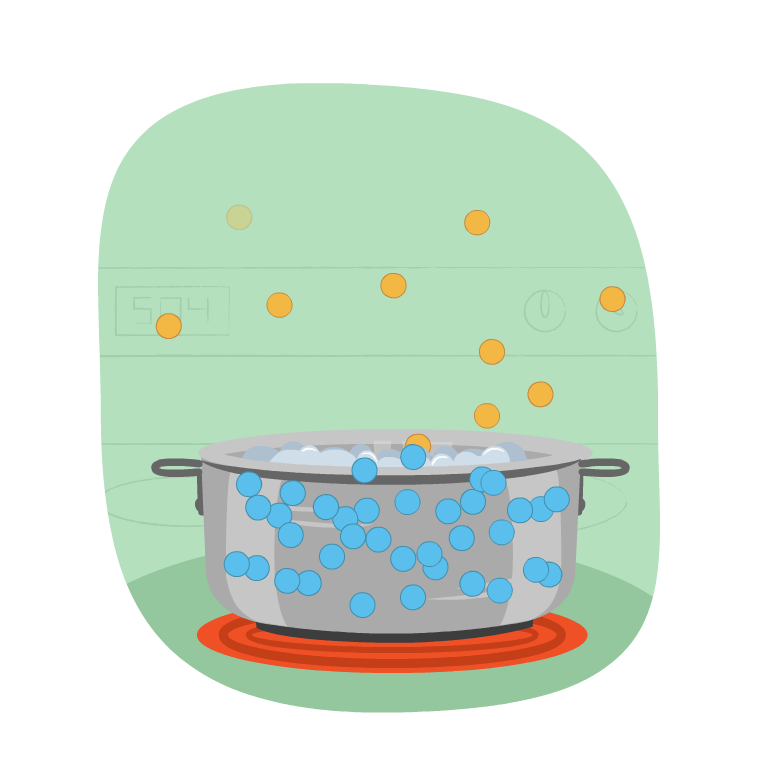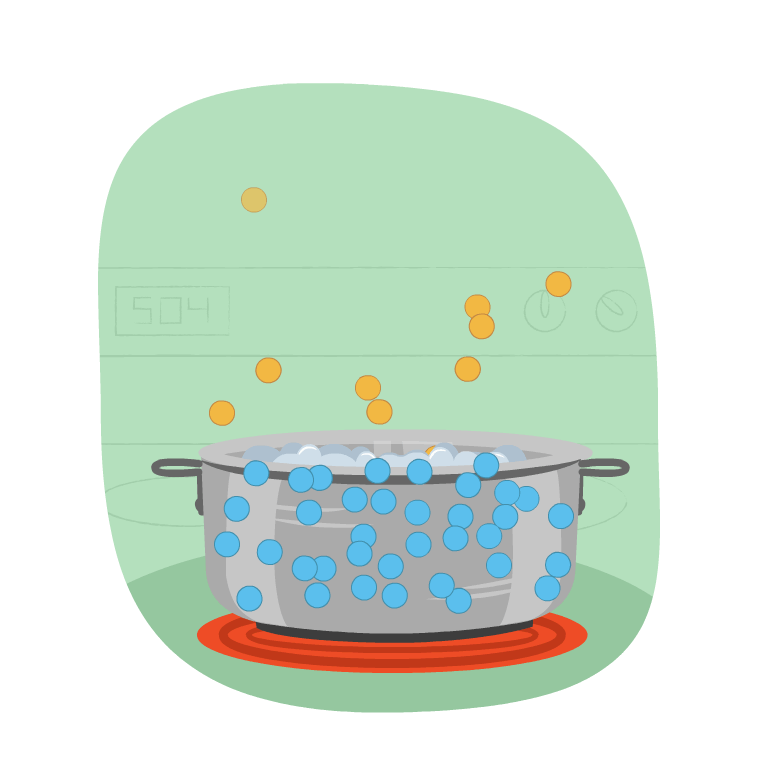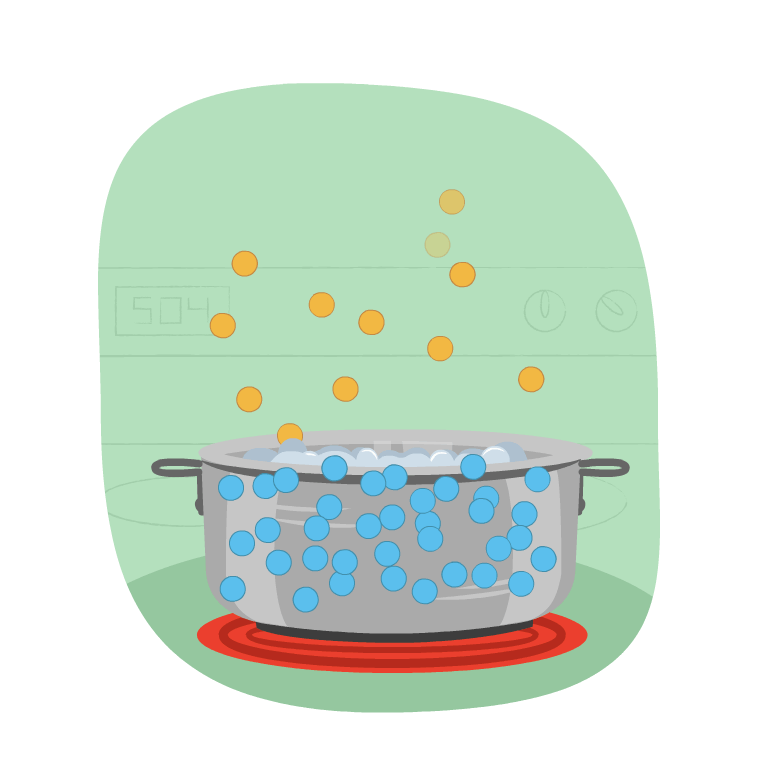
The correct time to add the rice is when the water starts to boil and releases steam.
This change in state is evaporation, which is the change of state from a liquid to a gas. When water is boiled, its temperature goes up and it changes into a gas.
Let’s go back to that hot summer day.
If Learner C took a swim in the pool and came out, depending on how hot it is, their swimsuit would dry because the water from it would evaporate into the air, due to the heat from the sun.
While Learner C is sitting by the poolside, they take a sip of their cold drink, which they notice is sweating! The moisture on the sides of their drink is called condensation.
What change in state of matter is this?
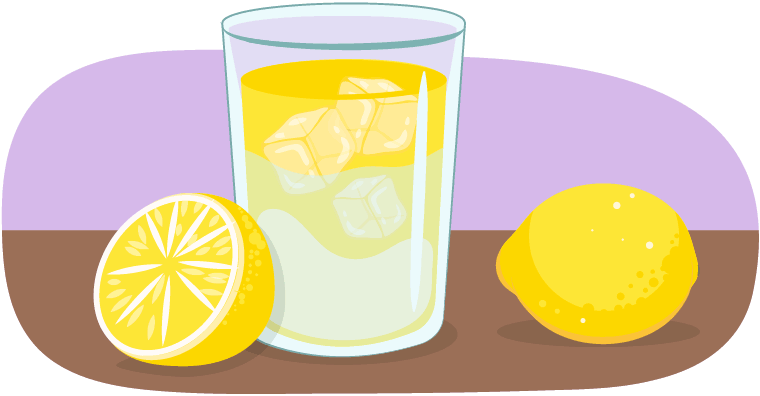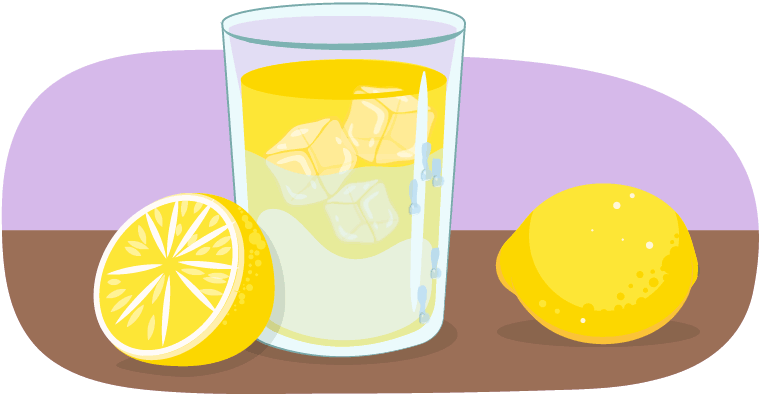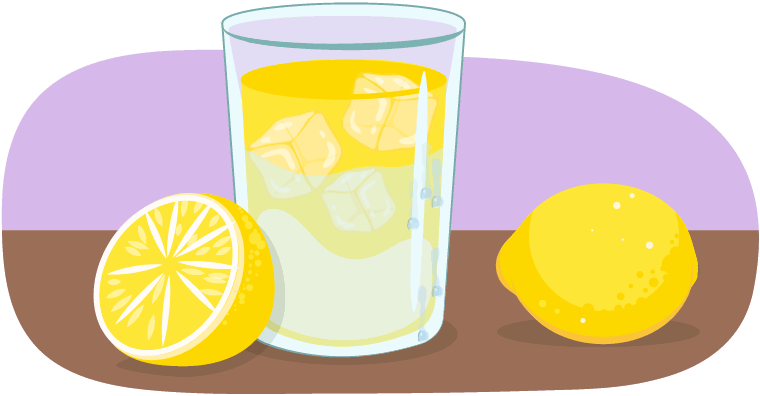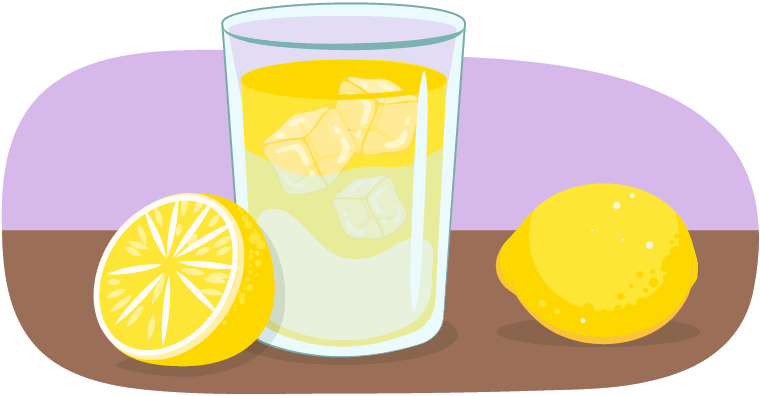
This is an example of gas turning into a liquid!
The water that is beading on the outside of the glass may look like the drink is leaking from the glass, but it is actually water vapour turning into liquid!
Water vapour is the gas state of water. It’s in the air all around us. When water vapour touches a cooler surface, like the ice-cold drink, it can condense into a liquid.
Let’s think of a few more everyday examples of where you might see the different changes in states of matter for melting, freezing, evaporation, and condensation.
Record your ideas in a notebook or another method of your choice.
Check your learning!
Before you move on, check your understanding using the fill-in-the-blanks activity!
For each sentence, select the missing word from the drop-down menu.
Try some more!
Sublimation

So far, you have explored different ways that matter changes its state. Most of these changes occur due to temperature and/or pressure. The water cycle is a perfect example of the changes of states of matter. Water (liquid state) that comes from rivers, lakes, streams, creeks, etc., evaporates (gas state) because of the heat of the sun. As the water droplets rise, the air gets cooler and they condense (liquid state) within the clouds until the water eventually falls from the clouds, called precipitation.
Depending on the temperature of the air, this precipitation may fall as rain or snow (liquid state) or sleet, ice, or hail (solid state).
As the precipitation falls and makes its way back into waterways, the cycle starts all over again. Explore the water cycle further in the following diagram.
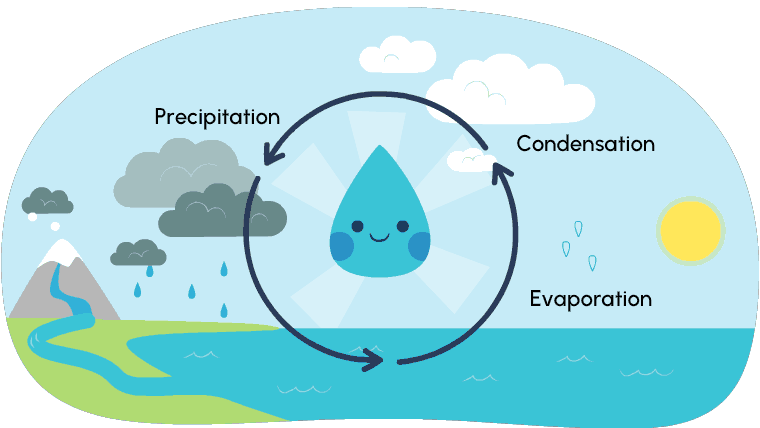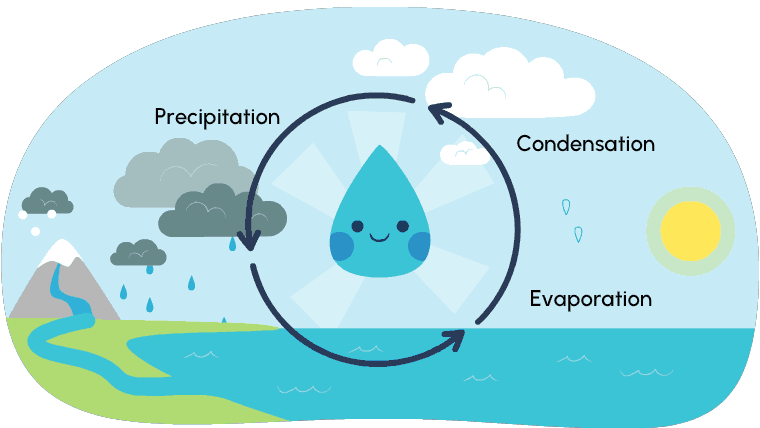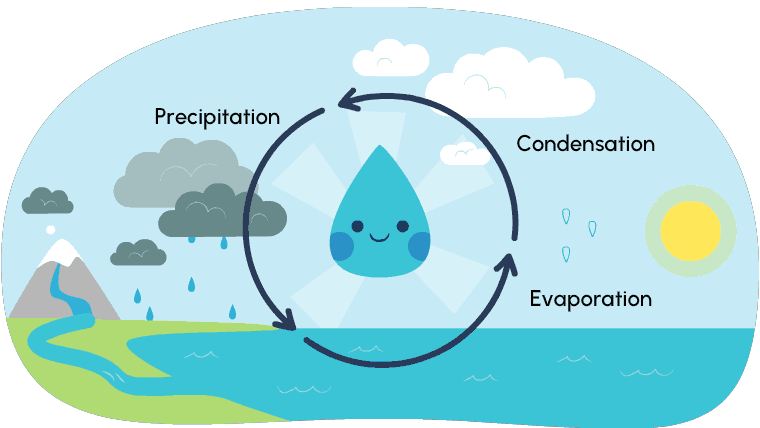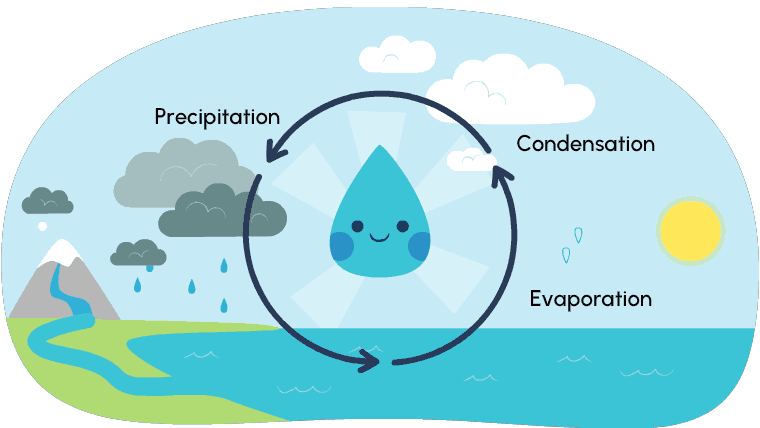
Solid, liquid, and gas are the three states of water. We experience water freezing and turning to ice, and water evaporating and turning to gas, but… have you ever explored ice (a solid) changing directly to a gas? This process is called sublimation.
Sublimation is the change of state from a solid to a gas without first becoming a liquid. It is most often used to describe the process of snow and ice changing into water vapour in the air without first melting into water.
The opposite of sublimation is vapour deposition, where water vapour (a gas) changes directly into ice (a solid). The most typical example of deposition is frost─ the deposition of water vapour from humid air (or air containing water vapour) onto a solid surface.

It is not easy to actually explore sublimation occurring, at least not with ice. One way to check out sublimation is to hang a wet shirt outside on a below-freezing day. Eventually the ice in the shirt will disappear. The best way to explore sublimation is to not use water at all, but to use carbon dioxide instead, specifically dry ice.

Dry ice is actually solid, frozen carbon dioxide, which happens to sublimate, or turn to gas, at a chilly -78.5 °C (-109.3°F). The fog is actually a mixture of cold carbon dioxide gas and cold, humid air, created as the dry ice “melts” or sublimates.
In your own words, what does sublimation and deposition mean when referring to water?
Press ‘Answer’ to access what sublimation and deposition means.
Sublimation is when ice evaporates directly into a gas and deposition is when water vapour changes directly into ice.
Experiment time!

Check out this video to learn about the steps of the Scientific Experimentation Process.
Safety
Before you explore the following experiment, let’s perform a safety check.
Hands-on Science
Ice cream science
For this experiment, we will be learning how to make ice cream.
Complete the Lab Sheet: Ice Cream Science in your notebook or using the following fillable and printable document. If you would like, you can use speech-to-text or audio recording tools to record your thoughts.
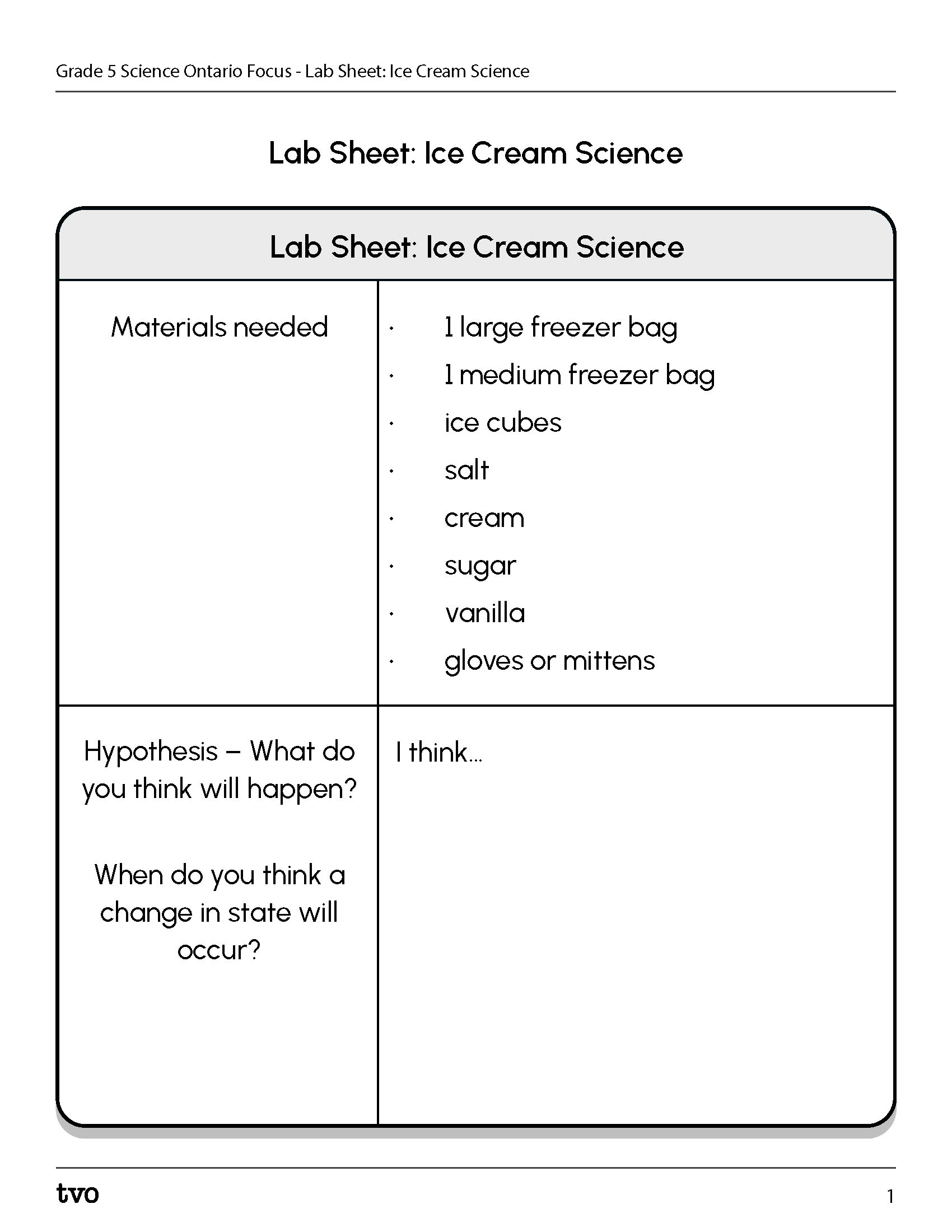
Press the Activity button to access the Lab Sheet: Ice Cream Science.
Activity (Open PDF in a new tab)Press the following tabs to access the materials and steps for the Ice Cream Science experiment.
If you do not have access to materials, access the “Video demonstration” tab to further explore changes to states of matter in action. You can use the video to make your observations and draw your conclusions.
- 1 large freezer bag
- 1 medium freezer bag
- ice cubes
- salt
- cream
- sugar
- vanilla
- gloves or mittens
- One cup of cream, one tablespoon of sugar, and ½ teaspoon of vanilla extract are added to a medium-sized freezer bag. The bag is sealed tightly.
- A large sized freezer bag is filled about ¾ full of ice, then ⅓ cup of salt is added.
- The smaller bag is placed inside the larger bag of ice and sealed.
- When possible, gloves or mittens are worn when handling the bag of ice. The bag is shaken hard for five minutes.
- The small bag is rinsed off with cold water before opening it to keep the salt water from getting into the ice cream.
And voilà! The homemade science ice cream is ready!
Explore this video to learn more about changes between states of matter.

Science is about reflecting and reimagining. Was your experiment successful?
Is there anything that you would change about your experiment design to improve it or the outcome?
Even if your experiment was not successful, what did you learn or confirm about the topic you were investigating?
Let’s reflect
- How did this experiment explore changes in states of matter?
- What do you think is the reason that salt is used in this experiment?
- Why is shaking the bag an important step of the process?
Press “Answer” to access the answers to the above questions.
- This experiment explores changes from the liquid state to the solid state. As the cream mixture begins to freeze, it changes from a liquid state to a solid state.
- Salt is an important part of this experiment because it helps to regulate the temperature. When salt and ice mix, the freezing point of the ice is lowered, and the freezing point reached depends on the amount of salt used. The more salt added, the lower the temperature can get before the saltwater solution freezes. By lowering the temperature at which ice freezes, we are able to create an environment in which the cream mixture could freeze at a temperature above 0℃ and become ice cream.
- The shaking moves the warmer cream mixture from the inside to the outside of the bag so it can freeze evenly. That way we can make a smoother product. It also adds air to the final product, so it’s fluffed up a little bit.
Consolidation
Learning check!

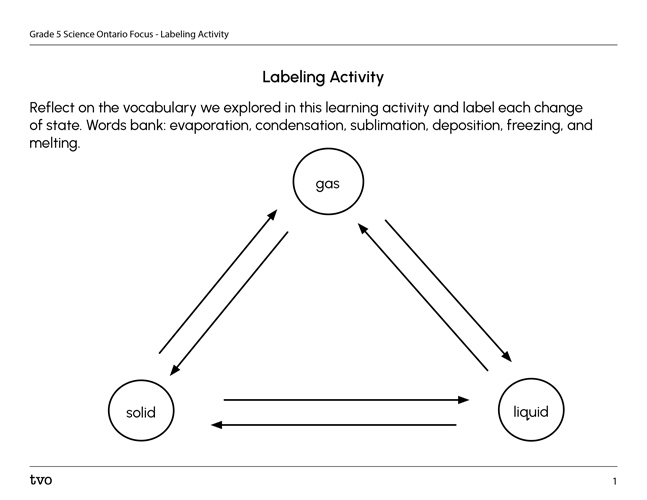
Reflect on the vocabulary we have explored in this learning activity and the changes of state of matter changes.
Complete the “Labeling Activity” in your notebook or using the following fillable and printable document. If you would like, you can use speech-to-text or audio recording tools to record your thoughts.
Reflect and connect
Based on your learning from this learning activity, choose two of the following questions to reflect on and make connections to your learning. Record your answers using a method of your choice.
- Based on the experiment, do you think all liquids turn to solid at the same temperature? Do you think all liquids turn to gas at the same temperature? Explain.
- Once something is a solid, does it stay that way forever? How do you know?
- Design your own experiment that demonstrates your knowledge of how solids, liquids, or gases change from one state to another. Be sure to include the materials you would need, step-by-step instructions, and how you would record observations.
- What are some everyday examples where you might observe the different changes in states of matter for melting, freezing, evaporation, and condensation?
- Use the water cycle to explain how matter changes state. Be sure to include key vocabulary, such as: precipitation, condensation, and evaporation.
Reflection
As you read through these descriptions, which sentence best describes how you are feeling about your understanding of this learning activity? Press the button that is beside this sentence.
I feel…
Now, record your ideas using a voice recorder, speech-to-text, or writing tool.